Understanding the Tendency of an Atom to Attract Electrons: A Deep Dive into Electronegativity and Its Implications
Guide or Summary:The Tendency of an Atom to Attract ElectronsUnderstanding ElectronegativityThe Role of Atomic StructureImpact on Chemical BondingApplicatio……
Guide or Summary:
- The Tendency of an Atom to Attract Electrons
- Understanding Electronegativity
- The Role of Atomic Structure
- Impact on Chemical Bonding
- Applications in Real-World Chemistry
**Translation of "the tendency of an atom to attract electrons":** the tendency of an atom to attract electrons
---

The Tendency of an Atom to Attract Electrons
The tendency of an atom to attract electrons is a fundamental concept in chemistry known as electronegativity. This property plays a crucial role in determining how atoms interact with each other, influencing the formation of chemical bonds and the behavior of molecules. Electronegativity is defined as the ability of an atom to attract shared electrons in a chemical bond. The greater the electronegativity of an atom, the stronger its ability to attract electrons.
Understanding Electronegativity
Electronegativity values are assigned based on a scale developed by Linus Pauling, where fluorine is the most electronegative element with a value of 4.0. Other elements have varying electronegativity values that dictate how they will behave in chemical reactions. For example, oxygen has an electronegativity of 3.5, while sodium has a much lower value of 0.9. This disparity explains why sodium readily loses an electron to form a positive ion, while oxygen tends to gain electrons to achieve a stable electron configuration.
The Role of Atomic Structure
The tendency of an atom to attract electrons is influenced by its atomic structure, particularly the number of protons in the nucleus and the distance of the electrons from the nucleus. Atoms with more protons have a stronger positive charge that can attract electrons more effectively. Additionally, the closer the electrons are to the nucleus, the stronger the attractive force they experience. This relationship is evident when comparing elements within the same group of the periodic table; as you move down a group, the electronegativity typically decreases due to increased distance between the nucleus and the outermost electrons.
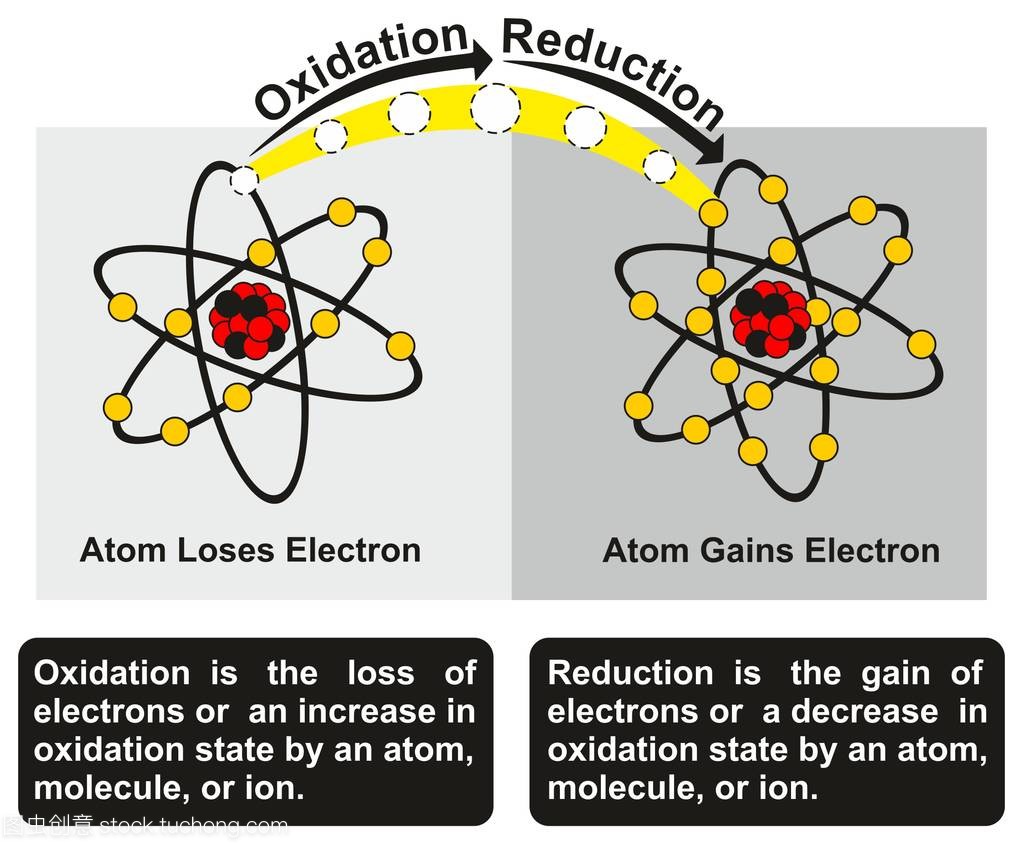
Impact on Chemical Bonding
The tendency of an atom to attract electrons significantly affects the type of chemical bonds that form between atoms. When two atoms with different electronegativities bond, the atom with the higher electronegativity will attract the shared electrons more strongly, resulting in a polar covalent bond. In contrast, if two atoms have similar electronegativities, they will share electrons equally, forming a nonpolar covalent bond. Understanding these interactions is essential for predicting the behavior of molecules in various chemical reactions.
Applications in Real-World Chemistry
The tendency of an atom to attract electrons has practical implications in various fields, including biochemistry, materials science, and pharmacology. For instance, the polarity of water molecules, which arises from the electronegativity difference between hydrogen and oxygen, is critical for its solvent properties and its role in biological systems. Additionally, the design of drugs often relies on understanding how different atoms will interact based on their electronegativity, allowing chemists to create compounds that can effectively target specific biological pathways.
In summary, the tendency of an atom to attract electrons, or electronegativity, is a key concept that underpins many aspects of chemistry and molecular interactions. By understanding this property, scientists can predict how different elements will behave in chemical reactions, how molecules will interact, and how to design new materials and drugs. The study of electronegativity continues to be a vital area of research, revealing deeper insights into the nature of chemical bonding and the behavior of matter at the atomic level.
