Understanding the Forces: Attractions Between Water Molecules Are Called Hydrogen Bonds
Guide or Summary:Introduction to Water MoleculesThe Nature of Hydrogen BondsImportance of Hydrogen Bonds in WaterEffects of Temperature on Hydrogen BondsHyd……
Guide or Summary:
- Introduction to Water Molecules
- The Nature of Hydrogen Bonds
- Importance of Hydrogen Bonds in Water
- Effects of Temperature on Hydrogen Bonds
- Hydrogen Bonds and Water's Role as a Solvent
**Attractions between water molecules are called** hydrogen bonds.
---
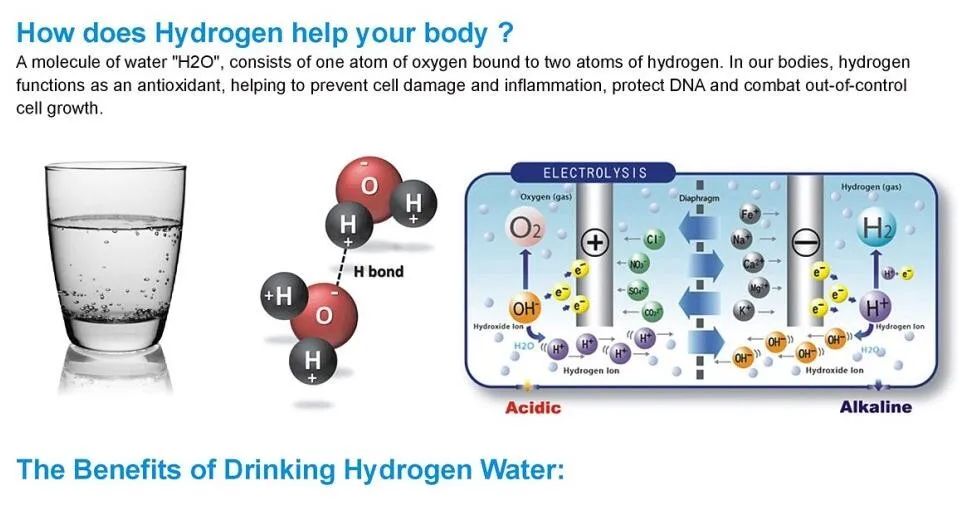
Introduction to Water Molecules
Water is a vital substance for all known forms of life, and its unique properties stem from its molecular structure. Each water molecule (H₂O) consists of two hydrogen atoms covalently bonded to one oxygen atom. This arrangement leads to an uneven distribution of electron density, creating a polar molecule with a partial negative charge near the oxygen atom and a partial positive charge near the hydrogen atoms.
The Nature of Hydrogen Bonds
The attractions between water molecules are called hydrogen bonds. These bonds are not as strong as covalent or ionic bonds but play a crucial role in determining the physical properties of water. A hydrogen bond occurs when the positive end of one water molecule (the hydrogen atoms) is attracted to the negative end of another water molecule (the oxygen atom). This interaction is responsible for many of water's unique characteristics, such as its high surface tension, boiling point, and solvent capabilities.
Importance of Hydrogen Bonds in Water
Hydrogen bonds contribute significantly to the cohesion and adhesion properties of water. Cohesion refers to the attraction between water molecules themselves, while adhesion is the attraction between water molecules and other substances. These properties are essential for various biological processes, such as the movement of water through plants (capillary action) and the formation of water droplets.

Effects of Temperature on Hydrogen Bonds
Temperature plays a critical role in the behavior of hydrogen bonds. As water is heated, the kinetic energy of the molecules increases, causing them to move more rapidly. This increased motion can break hydrogen bonds, leading to a decrease in water's density and causing it to expand. Conversely, when water cools, the molecules lose kinetic energy, and hydrogen bonds become more stable, leading to a denser liquid state. This unique behavior of water is why ice floats on liquid water, as the hydrogen bonds create a crystalline structure that is less dense than the liquid form.
Hydrogen Bonds and Water's Role as a Solvent
Water's ability to dissolve a wide range of substances is largely due to hydrogen bonds. When ionic or polar compounds are introduced to water, the water molecules surround the solute particles, forming hydrogen bonds with them. This interaction helps to separate the solute into individual ions or molecules, facilitating the process of dissolution. This property makes water an excellent solvent for biological reactions, allowing essential nutrients and waste products to be transported in living organisms.
In summary, the attractions between water molecules are called hydrogen bonds, and they play a fundamental role in the unique properties of water. Understanding these interactions helps us appreciate the significance of water in our environment and its essential role in sustaining life. From its high surface tension to its capacity as a universal solvent, hydrogen bonds are a key factor in the behavior of water, influencing everything from climate patterns to biological processes. As we continue to explore the complexities of water, the importance of hydrogen bonds will remain a central theme in both scientific research and everyday life.